Female Stress Urinary Incontinence
Workup
History
- Inciting factors: cough, sneeze, lifting, walk/run (PPV 73-74%, NPV 66-86%)
- Assess characteristics: chronicity, frequency/severity, treatment expectations, degree of bother, maximal pad usage
- Pad usage
- UTI/LUTS: urgency, frequency, nocturia, dysuria, hematuria, slow flow, hesitancy, incomplete emptying
- Pelvic symptoms: pain, pressure, bulging, dyspareunia
- GI symptoms: constipation, diarrhea, incontinence, splinting to defecate
- OB Hx: gravity, parity, delivery method, menopause status
- Prior SUI (non)surgical therapy
- PMHx: neurologic conditions, DM, dementia, prior radiation
- PSHx: prior pelvic surgery, SUI treatments
- Medications: stimulants, a-blockers, anticholinergics, diuretics
- Transient causes in elderly (DIAPPERS): delirium, infection, atrophic vaginitis, psychologic, pharmacologic, excess urine production, restricted mobility, stool impactino
- Fluid intake: water, alcohol, caffeine
- Questionnaires: currently no proven benefit
- Frequency/volume chart: can help differentiate SUI from OAB or other presentations
Physical
- Abdominal exam
- External genital/vaginal exam: lesions, tissue health, obvious bulges
- Urethra: check for masses, tendernes, and scarring, Q-tip test not predictive of SUI
- Cough stress test: 200mL in bladder has 84% sensitivity and 90% specificity, if no leak while recumbent can repeat while standing
- Vaginal vault: assess prolapse at rest and with valsalva, assess wall tenderness (pelvic floor dysfunction)
- Focused neuro exam: gait, congnitive status, lower extremity sensation/strength
- Rectal exam: assess for laxity, can assist with checking rectocele
- Bulbocavernosus reflex: assesses S2-4 function, present in 70% women and 100% men, squeezing glans causes anal and pelvic floor contractinos
Adjuncts
- Objective demonstration of SUI: positive stress test, involuntary urine loss while supine and/or standing
- 48hr pad test: PPV 81%, NPV 87%
- 1hr pad test: 94% sensitivity, 44% specificity, PPV 69%, 85% NPV
- Assess post-void residual: required, but single elevated PVR is not reliable
- Urinalysis: required, check for infection/hematuria
- Cystoscopy: optional, recommended if prior SUI surgery (especially if mesh used)
- UDS: not required if obvious SUI, optional for non-index patients
- Indications for further workup: unclear diagnosis, unable to demonstrate SUI, concern for neurogenic LUTS, abnormal UA (hematuria/pyuria), urge predominant MUI, elevated PVR, high grade PVR (3-4), significant voiding dysfunction
- Potential indications for further workup: OAB symptoms, prior failed surgery, prior prolapse surgery
Index vs Non-Index
- Index patient: otherwise healthy woman, interested in surgical therapy, SUI or stress-predominant MUI, no prior SUI surgery, low grade POP
- Non-Index Patient: POP stage 3-4, non-stress predominant SUI, incomplete emptying with elevated PVR, prior SUI surgery, mesh complications, high BMI, neurogenic LUTS, advanced age
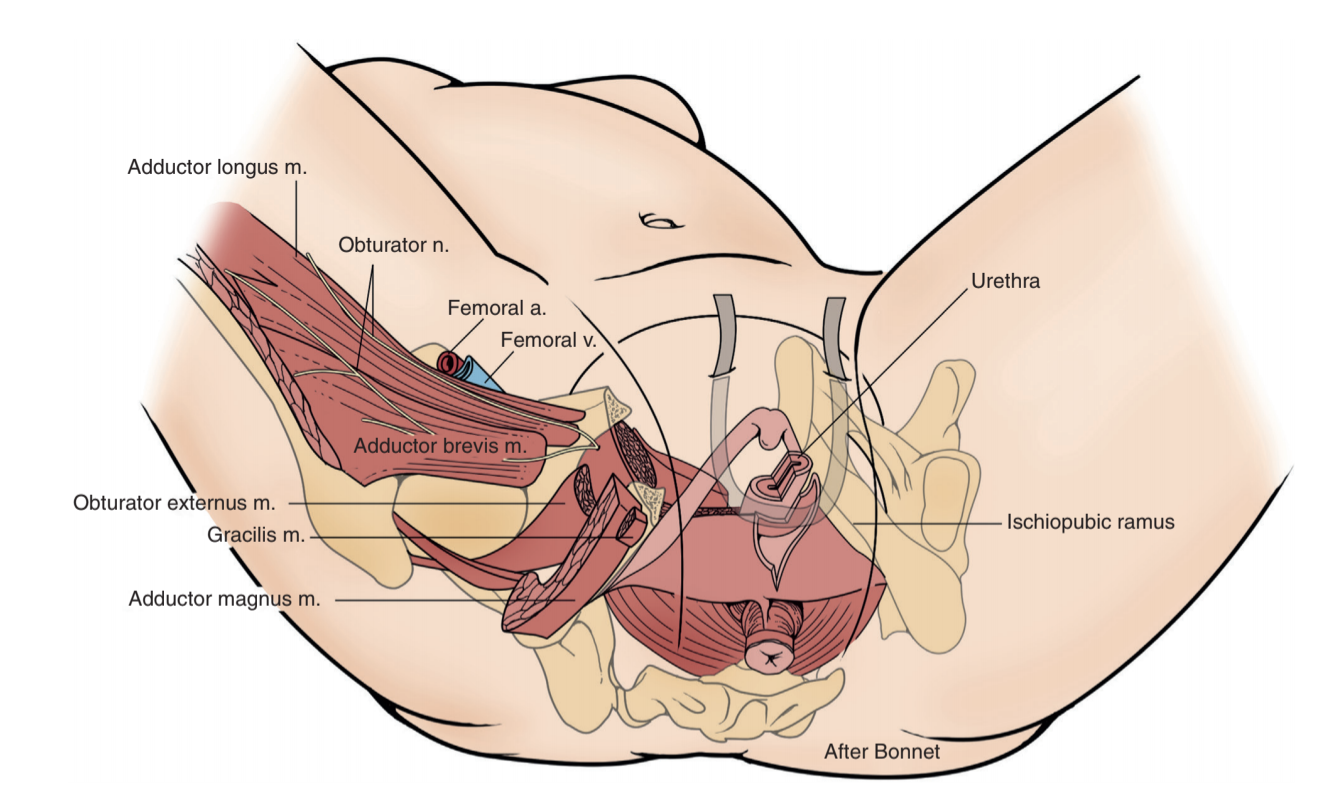
Retropubic sling location, from Campbell's

Transobturator sling placement, from Campbell's

Predicting SUI after POP surgery, from Jelovsek 2014
Treatment options
Non-Index Patient recommendations
- Intrinsic sphincteric deficiency (ISD): retropubic MUS, autologous PVS, and bulking agents - transobturator MUS does not provide adequate support with an immobile urethra
- Diverticulectomy, urethrovaginal fistula repair, or mesh excision: avoid mesh placement at same time (high risk for erosion), but staged procedure is okay, can use PVS
- History XRT, scarring, or poor tissue quality: avoid mesh placement (high risk for erosion)
- Pelvic prolapse repair: all options available, see nomogram for probability of needing concurrent SUI procedure
- NGB: factor in patient-specific risks/benefits, do not use mesh if requiring catheter
- Prepregnancy: high rate of SUI recurrence but all options are available (including synthetic sling)
- Diabetes: increased risk of mesh erosion but can use any option
- Obesity: increased BMI decreases overall effectiveness, but can use any option
- Age > 65yrs: overall lower success rates
Sling facts
- Anatomy: creates a suburethral hammock to prevent urethral hypermobility, requires pubourethral ligaments, suburethral vaginal hammock, and pubococcygeus muscles for support
- Types: "midurethral" refers to FDA-approved synthetic slings (retropubic or transobturator), whereas "pubovaginal" refers to autologous fascial slings
Midurethral Sling
- Retropubic: placed via suprapubic incision
- Transobturator: placed via lateral incision
- Technique: goal is urethral support (loose sling), not urethral kinking (tight sling)
- Retropubic risks: vascular/visceral injuries, bladder/urethral perforation, voiding dysfunction, suprapubic pain
- Transobturator risks: groin pain, need for repeat surgery
- Do not place if: fistula, prior mesh erosion, intraoperative urethral injury, or diverticulum
Pubovaginal sling
- Location: placed at bladder neck, not at midurethra
- Fascial harvest: autologous rectus or fascia lata, worse outcomes with non-autologous fascia, but increases perioperative morbidity (26% wound complications) and postoperative pain, erosino risk nonexistent with autologous tissue
- Success: 24-97% continence, 3-7% reoperation/revision, new urgency 2-22% (not predictable preop)
- Postoperative voiding dysfunction: initially manage conservatively, manage complete retention with sling loosening via inferior pressure from rigid cystoscope, perform complete urethrolysis (success 65-93%), incision (success 84-100%)
Colposuspension
- Indications: useful if undergoing A/P surgery at the same time (hysterectomy etc), avoids mesh and fascial harvest complications, but 20% have wound complications
- Burch technique: permanent sutures placed through anterior vaginal wall next to urethra/bladder neck and suspended to ileopectineal (Cooper) ligament
- Marshall-Marchetti-Krantz (MMK): permanent sutures placed through periurethral tissue and sutured to periostoeum or cartilage of symphysis pubic
- Side effects: urge incontinence (9-13%), UTI (32%), voiding dysfunction (3-9%), bladder injury (1-4%), osteitis pubic (2.5%, MMK only)
Intraurethral bulking agents
- Inject material into submucosal space to coapt urethra
- Less invasive with shorter operative and recovery time
- Current options: bovine collagen, zirconium beads (durasphere), silicone (macroplastique), calcium hydroxylapatite (Coaptite), polyacrylamide (Bulkamid)
- Visible on X-ray: Durasphere, Coaptite, Teflon
- Can be performed via periurethral or transurethral approach
- May require repeat injections
- Success: 30-40% completely dry at 1yr, 50-60% dry or improved
- Side effects (transient): retention (0-12%), elevated PVR (55-75%), storage symptoms (17-46%), hematuria (8-40%), dysuria (9-46%), UTI (0-23%)
- Retention management: short course catheterization, concern for bulking agent molding around catheter
- Rare complications: periurethral abscess, urethral necrosis, urethral erosion, bulking agent extrusion, particle migration
- Contraindications: UTI, inflammation, friable urethra, stricture, pregnancy, hx pelvic XRT
Nonsurgical options
- Behavioral: timed voiding, voiding before exercise, pelvic floor PT
- can be inserted intraurethral or intravaginal, help to obstruct urethra, may cause bladder spasms
- Pessary: has knob to compress urethra, also treats prolapse
- Medication: currently no recommended options for treatment of stress incontinence
Sling complications
General surgical risks
- Pain: abdominal, pelvic, vaginal, groin, thigh, dyspareunia
- Bleeding: need for transfusion is rare
- Infection: UTI rates highest within first 3mo
- Intraop injury: do not place mesh sling if urethral injury
- Mesh: exposure, erosion, perforation
- LUTS: may unmask or worsen OAB symptoms, although 50% see improvement in urge symptoms after sling placement
Vaginal mesh exposure
- Prevalence: 0.5-8.1%
- Symptoms: vaginal discharge, rough vaginal surface, dyspareunia, pelvic pain, UTI symptoms
- Management: observation, estrogen +/- antibiotic cream, limited excision and trimming with reapproximation, or liberal mesh excision
Urethral perforation
- Prevalence: 0-0.6%
- Intraoperative management: repair and do not place mesh sling at that time
- Postoperative presentation: presents with voiding/storage symptoms, retention, rUTI, and incontinence
- Diagnosis: visualize mesh in urethra on cystoscopy
- Management: consider endoscopic excision (may require multiple procedures) vs transvaginal urethrotomy and excision
Bladder perforation
- Prevalence: 0.5-0.6%, usually caused by unrecognized intravesical mesh placement, more common with retropubic (4.8%) than transobturator (0.6%)
- Intraoperative diagnosis: assess full bladder intraoperatively with 30 and 70 degree lenses, can remove trocar and replace if identified
- Presentation: abdominal pain, hematuria, rUTI, LUTS, dysuria, incontinence
- Diagnosis: use cystoscopy, imaging not adequate
- Management: endoscopic incision vs transvaginal or retropubic mesh excision
Pain/Infection
- Risks: younger age, preexisting pelvic pain, and prior chronic pain diagnosis
- Prevalence: 16% after transobturator vs 1.5% after retropubic, 0-2% vaginal wound infections
- Management: NSAIDs, rest, and PT, rarely requires excision with orthopedic assistance, can consider immediate removal of mesh prior to tissue ingrowth
- Outcomes: per Zeng 2021, improved pain resolution if complete (vs partial) removal, no difference in SUI recurrence or reintervention if partial vs complete removal
Voiding dysfunction
- Prevalence: 2% after retropubic vs 0% after transobturator (retention seen 3.7% vs 0%), 8% require temporary catheterization after surgery
- Diagnosis: VCUG, translabial US, MR pelvis
- Sling readjustment: give 3mo after PVS or 1mo after MUS before performing incision, irreversible bladder remodeling can occur within 6mo
References
- AUA Core Curriculum
- Boone, T., J. Stewart, and L. Martinez. "Additional Therapies for Storage and Emptying Failure." Campbell-Walsh Urology 12 (2020).
- Cameron, A. "Complications Related to the Use of Mesh and Their Repair." Campbell-Walsh Urology 12 (2020).
- Gomelsky, A. and R. Dmochowski. "Slings: Autologous, Biologic, Synthetic, and Mid-urethral." Campbell-Walsh Urology 12 (2020).
- Jelovsek, J. Eric, et al. "A model for predicting the risk of de novo stress urinary incontinence in women undergoing pelvic organ prolapse surgery." Obstetrics and gynecology 123.2 0 1 (2014): 279.
- Kobashi, Kathleen C., et al. "Surgical treatment of female stress urinary incontinence: AUA/SUFU guideline." The Journal of urology 198.4 (2017): 875-883.
- Lucioni, A. and K. Kobashi. "Evaluation and Management of Women with Urinary Incontinence and Pelvic Prolapse." Campbell-Walsh Urology 12 (2020).
- Wieder JA: Pocket Guide to Urology. Sixth Edition. J.Wieder Medical: Oakland, CA, 2021.
- Zeng, Jiping, et al. "Symptom Resolution and Recurrent Urinary Incontinence Following Removal of Painful Midurethral Slings." Urology 159 (2022): 78-82.