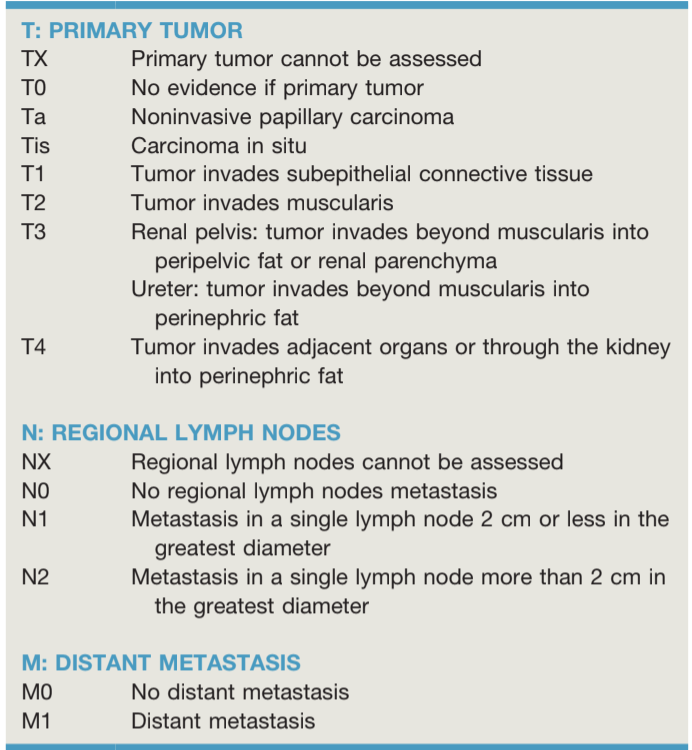
Upper Tract Urothelial Cancer
Background
UTUC by the numbers
- Bilateral: 1-5%
- Multifocal: 10-20%
- History bladder cancer: 4-41%, depending on population
- Disease recurrence: 22-47% in bladder, 2-6% in contralateral renal unit
- Spread: 60% are invasive, 7% are metastatic
Risk Factors
- Lynch syndrome (HPNCC): suspect if UTUC and age < 60, or first degree relatives x2 with HPNCC-related cancers (colorectal, ovarian, endometrial, gastric, biliary, small bowel, pancreatic, prostate, skin, and brain cancer), Amsterdam II criteria (3+ relatives w/ HPNCC cancer, 2+ generations affected, 1+ tumors before 50yo)
- Tobacco: increases risk 2.5-7x
- Occupational: exposure to aromatic amines increases risk 4-5.5x
- Aristolochic acid (Chinese herb) nephropathy: Aristolochia fangchi produces a compound that induces p53 mutation, resulting in ESRD and UTUC
- Arsenic: associated with UTUC and peripheral vascular disease (blackfoot disease)
- Inflammation: chronic upper tract irritation from stones or UTI increases cancer risk
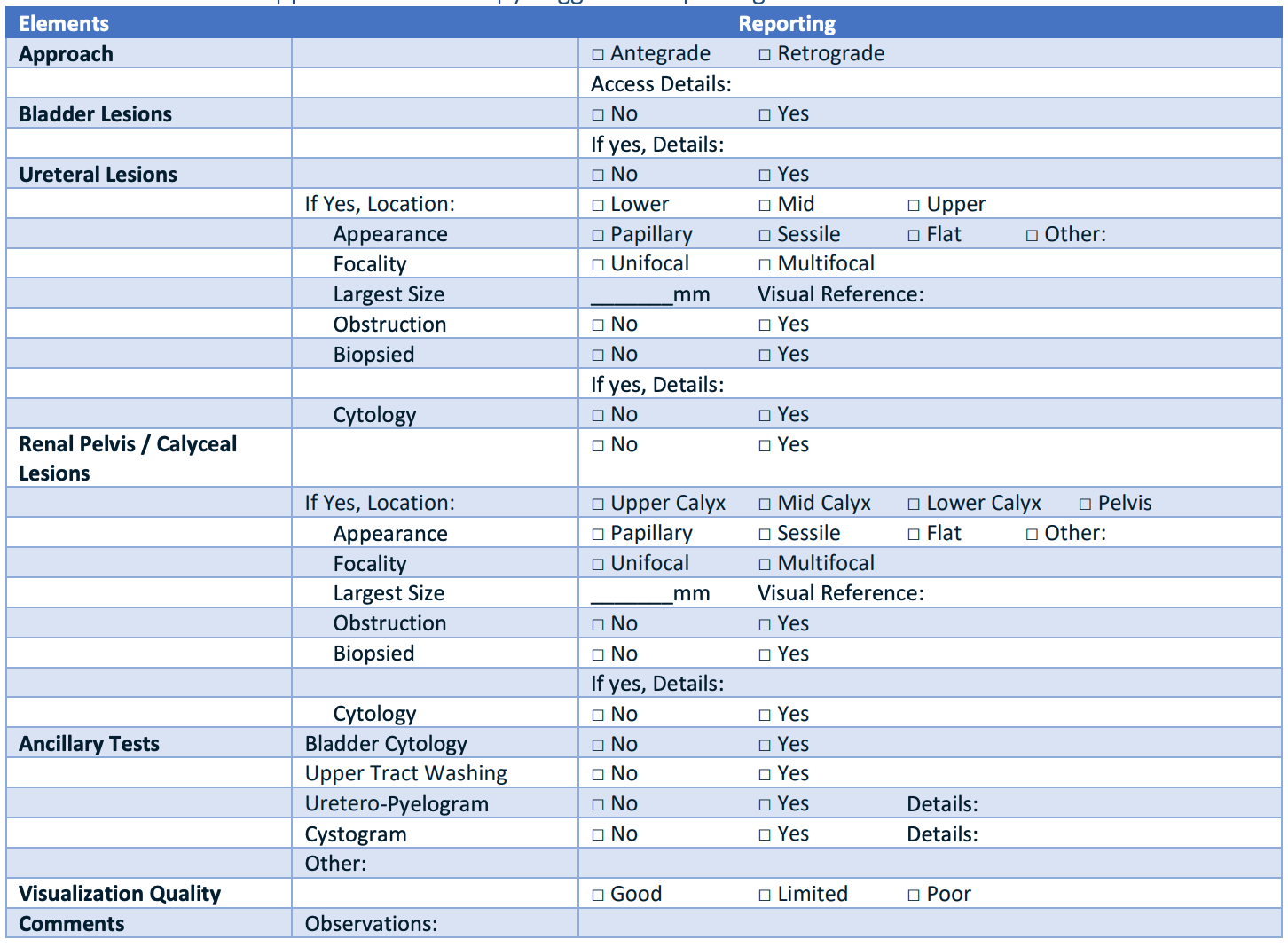
Standard ureteroscopy reporting, from AUA Guidelines
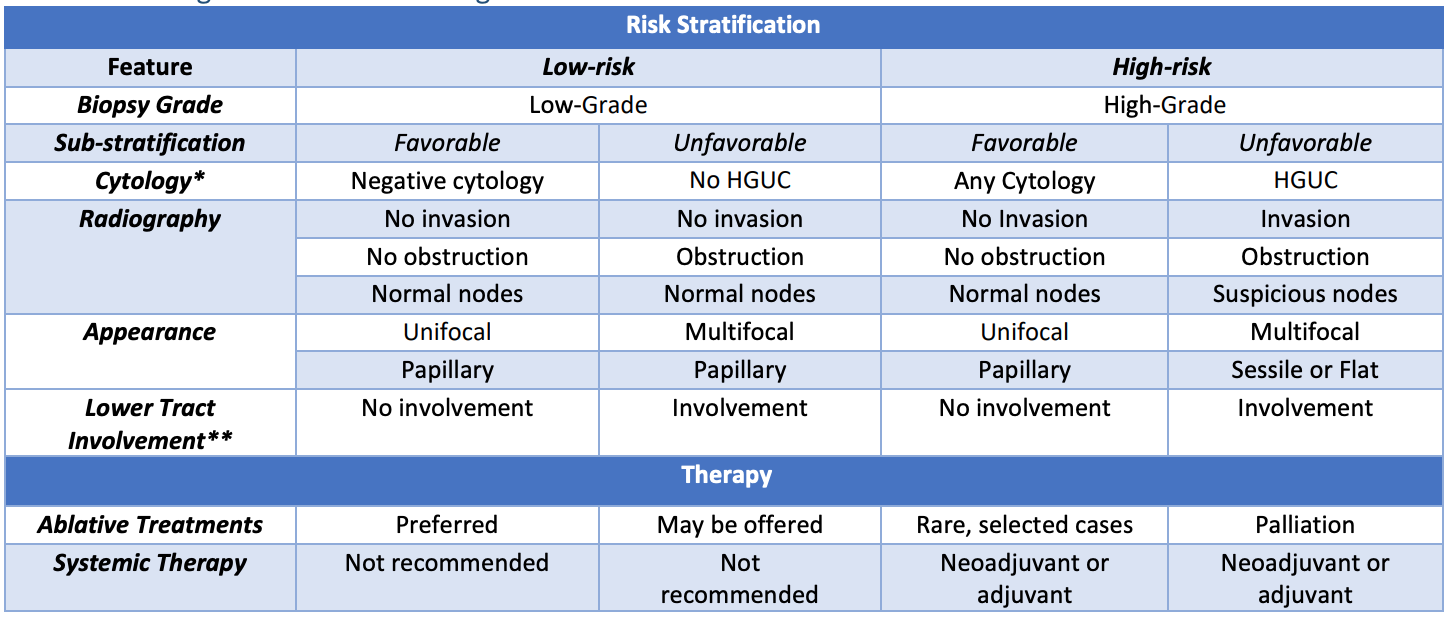
Risk stratification, from AUA guidelines
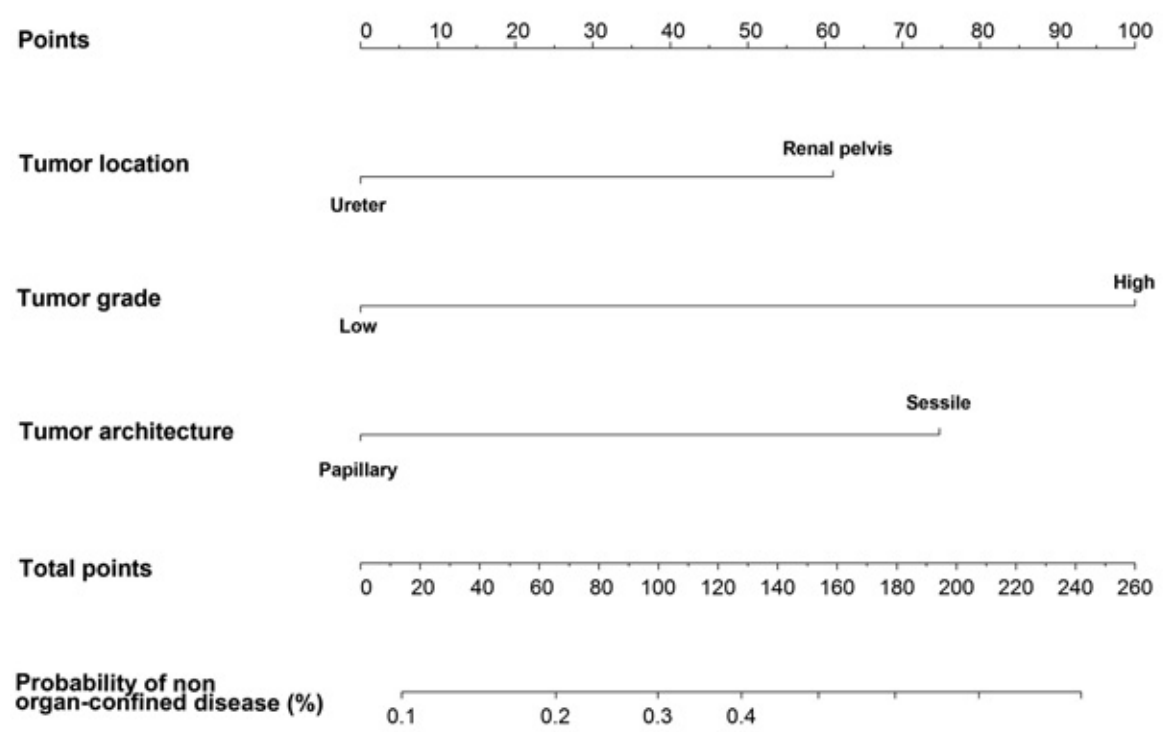
Probability of non-organ confined disease, from Margulis et al
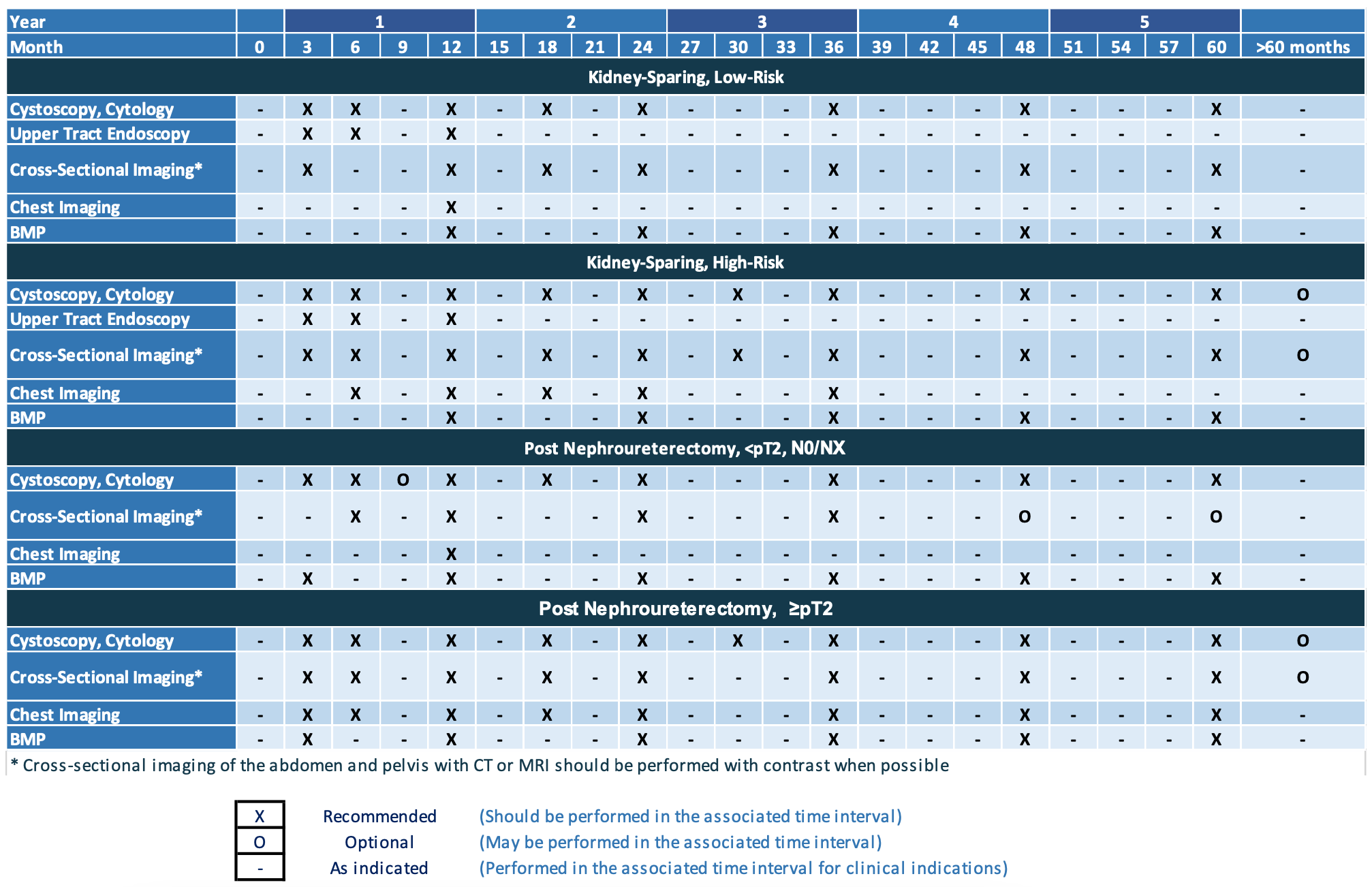
Follow-up after initial management, from AUA guidelines
Initial Evaluation
Workup
- Symptoms: hematuria (56-98%), flank pain (20-30%), lumbar mass (10%) - incidental diagnosis (15%)
- Initial evaluation: cystoscopy (asssess for synchronous bladder cancer) and upper tract imaging (CT/MR), ultrasound not recommended for initial evaluation
- Hydronephrosis: seen with invasive ureteral tumors in 80%
- Positive lymph nodes: high specificity (91%) vs low sensitivity (25%) for pathologic disease, consider systemic treatment if concern for LN+ disease prior to surgery (per Pallauf 2023)
- Further imaging: obtain only if concern for high-grade or invasive disease - obtain chest imaging, bone scan and brain imaging on select patients
- Genetic testing: 7-20% have Lynch syndrome, genetics referral recommended if < 60yo, personal/family history of UTUC or Lynch syndrome cancers
Endoscopic diagnosis
- Ureteroscopy: recommended unless diagnosis can be made from combination imaging and cytology, do not perform if biopsy will not affect management (expectant management), tissue diagnosis relatively accurate (Sens 83-91%, Spec 88-100%), can be performed antegrade if necessary (potential risk of tract seeding)
- Ureteroscopy risks: stricture (5-25%, more common with cautery than laser), perforation (uncommon), seeding (rare)
- Cytology classification (Paris System): nondiagnostic, negative for HG cells (NHGUC), atypical (AUC), suspicious (SHGUC), HGUC, low-grade urothelial neoplasm (LGUN), and other malignancies
- Recurrence after endoscopic complete resection: 30-50% at 5yr, 20-30% will require NUx
Ureteroscopy tips
- Biopsy options: use stone basket or forceps (Piranha, BIGopsy), brush, laser (with basket), or loop resection if performing antegrade
- Unable to access tumor: consider pre-stenting and returning, consider cytology only (collect prior to retrograde pyelogram), consider antegrade access
- Synchronous bladder tumor: resect at same time as upper tract lesion
- Contralateral ureteroscopy: not recommended if imaging otherwise negative - potential risk for seeding
Post-diagnosis evaluation
- Risk group assessment: see table from AUA guidelines to help determine best treatment option
- Nephrology evaluation: potential postoperative risk for CKD, consider if baseline GFT < 45 or patient has proteinuria
Organ-sparing treatments
Endoscopic ablation
- Absolute indications (all must be present): low grade pathology, no high grade cytology, papillary (not sessile), tumor < 1.5cm, solitary tumor, no invasion on iamging, no variant histology, no hydronephrosis, no prior cystectomy for HG bladder cancer
- Relative indications (not required): poor renal function, solitary kidney, poor surgical candidate, bilateral tumors, favorable HR or unfavorable LR with low tumor burden (< 1.5cm)
- Tips: can perform antegrade/retrograde, assess for recurrence at 3 months
- Adjuvant intraluminal chemotherapy: evidence extrapolated from bladder cancer data, can instill via nephrostomy tube, ureteral catheter, or into bladder with double-J stent
Jelmyto (Mitomycin hydrogel)
- Indications: Ta, T1, or CiS
- Technique: can administer antegrade/retrograde, stays in kidney then dissolves (reverse thermal gel), administer weekly for 6 weeks, 58% showed complete response at 3mo (56% sustained response at 12mo)
- Tips: wait 3-4wks after endoscopic surgery, do not perform if perforation, check CBC each time, alkalinize urine (1.3g NaBicarb night before and morning of), measure renal pelvis volume with contrast
- Side effects: ureteral stenosis/stricture (44%), UTI (32%), hematuria (31%), flank pain (30%), nausea (24%), myelosuppression (3%), contact dermatitis
Padeliporfin (TOOKAD)
- Mechanism: "vascular-targeted photodynamic therapy" - give medication IV, exposing tumor to certain laser frequency via ureteroscope causes cancer cell death
- Outcomes: 73% complete resopnse, no specific side effects from treatment (other than stent/URS side effects)
BCG
- Indications: HR favorable disease after complete resection or CiS
- Efficacy: questionable, depends on the study
- Side effects: cystitis, stricture, sepsis, renal TB, stricture, pericarditis
Watchful waiting
- Indications: significant comorbidities/risk of mortality, high risk for ESRD with any interventions
- Symptomatic progression: bleeding, obstruction, infection, pain - palliative options may be limited when these occur
Excisional surgeries
Nephroureterectomy
- Indications: HR disease and able to tolerate surgery
- Principles: complete excision of ipsilateral urothelium, removal of bladder cuff, avoidance of urinary spillage,
- Bladder cuff: decreases recurrence risk (otherwise 16-58%), excisional technique preferred over transurethral (higher recurrence risk, more difficult to give intravesical chemotherapy if bladder not closed)
- Nephroureterectomy with bladder cuff is gold standard for any high-grade or invasive lesions, prevents ipsilateral recurrence (15-44%), low risk for contralateral recurrence (< 8%)
- Intravesical chemotherapy: give single dose mitomycin or gemcitabine immediately postoperatively to decrease risk for bladder cancer
- Not taking bladder cuff: recurrence risk 16-58%
- 5yr survival after nephroureterectomy: Ta/T1 60-90%, T2 43-75%, T3 16-33%, T4 0-5%, N1+ 0-4%, M1+ 0%
Distal ureterectomy with reimplant
- Indications: tumor isolated to lower-third of ureter, bladder mobile with good capacity for reimplant
- Tips: use only for distal third of ureter, unclear benefit to non-refluxing reimplant
Segmental ureterectomy
- Indications: < 1cm tumor requiring resection of < 2cm
- Tips: reanastomose over a stent, avoid tumor spillage, send frozen margin to confirm prior to anastomosis
Other surgical options
- Partial nephrectomy: need to be able to remove portion of collecting system without urine leak
- Ureterectomy + ileal ureter: poor candidate for segmental ureterectomy, has higher risk for complications
Lymph node dissection
- Indications: high grade, high risk, potentially invasive
- Volume: recommended to remove 8+ nodes to confirm pN0 status
- Renal pelvis tumor template: remove from renal vein to at least IMA and overlying ipsilateral great vessel
- Proximal/middle ureter template: remove from renal vein to aortic bifurcation and overlying ipsilateral great vessel
- Distal ureter template: remove external iliac and obturator nodes, remove internal and common iliac nodes as indicated
Non-Surgical Treatments
Peri-Surgical Chemotherapy
- Neoadjuvant chemo: complete response rate 14-19%, indicated if postoperative eGFR will be < 60 or other postoperative factors will prevent receiving cisplatin-based chemotherapy
- Perioperative intravesical chemo: instill at time of surgery or up to 48 hours afterwards, can use mitomycin or gemcitabine, per ODMIT-C trial (O'Brien et al), 1 dose mitomycin immediately postoperatively decreased bladder cancer risk from 27% to 17% at 1yr
- Adjuvant chemo: indicated if no neoadjuvant chemotherapy and pT2-T4 or N1-N3, per POUT trial (Birtle et al) it improves disease-free survival (HR=0.48 at 30mo), can use gem/cis (GFR > 50) or gem/carbo (GFR < 50)
Adjuvant nivolumab
- Indications: neoadjuvant chemo was given and path shows ypT2-4 or ypN+ or patients who are ineligible/refuse adjuvant cisplatin
- Side effects: pruritus (23%), fatigue (17%), diarrhea (17%), elevated lipase/amylase (3-5%), colitis (1%), pneumonitis (1%)
Metastatic disease (cN1-3 or c/pM+)
- Management: systemic chemotherapy over up-front surgery, consider surgery adequate radiographic response
- XRT: indicated only for symptom control
References
- AUA Core Curriculum
- Coleman, Jonathan A., et al. "Diagnosis and Management of Non-Metastatic Upper Tract Urothelial Carcinoma: AUA/SUO Guideline (2023)." (2023).
- Jarret, T., S. Matin, and A. Smith. "Surgical Management of Upper Urinary Tract Urothelial Tumors." Campbell-Walsh Urology 12 (2020).
- Kallidonis, P., and E. Liatsikos. "Urothelial Tumors of the Upper Urinary Tract and Ureter." Campbell-Walsh Urology 12 (2020).
- Margulis, Vitaly, et al. "Preoperative multivariable prognostic model for prediction of nonorgan confined urothelial carcinoma of the upper urinary tract." The Journal of urology 184.2 (2010): 453-458.
- Pallauf, Maximilian, et al. "Diagnostic accuracy of clinical lymph node staging for upper tract urothelial cancer patients: a multicenter, retrospective, observational study." The Journal of urology 209.3 (2023): 515-524.
- Wieder JA: Pocket Guide to Urology. Sixth Edition. J.Wieder Medical: Oakland, CA, 2021.
- Woldu, Solomon L., Aditya Bagrodia, and Yair Lotan. "Guideline of guidelines: non‐muscle‐invasive bladder cancer." BJU international 119.3 (2017): 371-380.