Testis Cancer
Background
Epidemiology
- Prevalence: rare, only 8K-9K cases per year
- Age: most common cancer in 20-40yo men
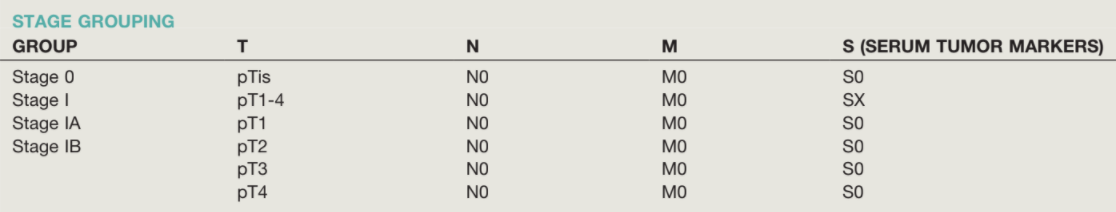
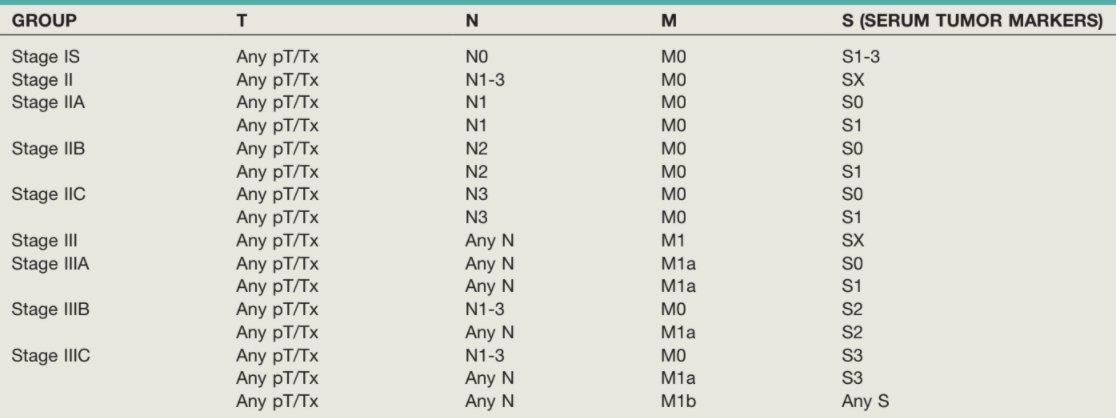
- Stage at presentation: majority (~2/3) present with Stage I, < 15% present with Stage III disease
- Bilateral disease: 2%, usually metachronous within 5yrs of initial presentation
- Extragonadal primary: seen in 5%, usually in retroperitoneum or mediastinum
Risk factors
- Intratubular germ cell neoplasia (ITGCN): 50% testis cancer risk in 5yrs
- Cryptorchidism: 4-6x, 2-3x if pexied pre-puberty, 1.74x risk in contralateral testis
- Family hx: increased risk if first-degree relative with testis cancer
- Personal hx: 12x compared to general population, but 15yr incidence is only 2% (because testis cancer is overall rare)
- Other potential risks: HIV, gonadal dysgenesis with Y chromosome, infertility (2-3x risk), Klinefelter syndrome (increased extragonadal risk), CHEK2 mutation (4x risk)
- Non-risk factors: atrophy, trauma (increases chances of diagnosis), microlithiasis (incidental finding only)
Types
- Germ cell tumors: seminoma (~55%) vs non-seminoma germ cell tumor (NSGCT, ~45%)
- NSGCT: includes choriocarcinoma, yolk sac, embryonal, and teratoma
- Seminoma: doesn't make AFP, XRT-sensitive
- Choriocarcinoma: can spread hematogenously, cause hemorrhagic metastases
- Teratoma: chemo-resistant, can rapidly grow, can transform into somatic malignancies (sarcoma, etc), no difference in adults between immature and mature
- Embryonal carcinoma: presence in orchiectomy specimen increases risk for occult metastases
- Yolk sac tumor: more common in mediastinal and pediatric tumors, almost always make AFP, do not make HCG
Presentation and Workup
Symptoms/Signs
- Symptoms: painless swelling, pain (10% from hemorrhage/infarction), chest/back/abdominal pain, cough/dyspnea
- Signs: hard/enlarged testicle, abdominal mass, subclavicular lymphadenopathy, gynecomastia
Imaging workup
- Scrotal US: solid testis mass is testis cancer until proven otherwise
- Staging: obtain CT C/A/P to assess for retroperitoneal and other lymphadenopathy, CT misses 30% metastatic disease
- Chest imaging: can get CXR instead of CT chest if low risk for thoracic metastases
- Brain imaging: consider if HCG > 5K, AFP > 10K, neurologic symptoms, extensive lung mets, or non-pulmonary visceral mets
- Bone imaging: only if specific clinical indication
- Observation: if mass < 1cm and normal STMs, can consider repeating labs and US in 6-8 weeks (may be benign lesion), consider MRI for inconclusive lesions
Serum Tumor Markers (STMs)y
- LDH: nonspecific marker produced by 20% GCT, magnitude correlates w/ disease bulk, also seen with lymphoma and infection/inflammation
- AFP: produced by yolk sac and embryonal (elevated AFP = NSGCT), never produced by pure choriocarcinoma or seminoma, also seen with infants < 1yo, lung, biliary, stomach, panc, liver cancer, liver disease, ataxia telangiectasia, and tyrosinemia
- bHCG: produced by 10-30% NSGCT (always if choriocarcinoma present) and 10-15% seminomas, also seen with liver, panc, stomach, biliary, lung, breast, kidney, bladder cancer, marijuana use, hyperthyroid hypogonadism (cross-reactive with LH)
- Do not treat solely based on elevated LDH (non-specific) or elevated AFP < 25 (can be normal)
- Half-Life: AFP (5-7 days), LDH (3-5 days), bHCG (24-36 hours)
- S0: normal STMs
- S1: LDH < 1.5x normal, AFP < 1K, bHCG < 5K
- S2: LDH 1.5-10x normal, AFP 1-10K, bHCG 5-50K
- S3: LDH > 10x normal, AFP > 10K, bHCG > 50K
Sperm Banking
- Discuss sperm banking prior to orchiectomy (standard of care)
- Pre-orchiectomy findings: 50% have abnormal semen parameters and 10% have azoospermia at presentation
- Cisplatin chemotherapy: causes azoospermia, 50% recovery by 2yrs, 80% by 5yrs
- XRT: > 6 Gy causes azoospermia, oligospermia is dose dependent below 6 Gy
- RPLND: 80% have permanent ejaculatory dysfunction, reduced to 10% w/ nerve-sparing techniques
Orchiectomy
Tips for Minimizing Cancer Recurrence
- Approach: perform orchiectomy via inguinal (not scrotal) incision (standard of care), removes spermatic cord and does not alter lymphatic drainage
- Local recurrence rates 2.5% with scrotal violation (vs 0% via inguinal approach)
- If able, place testis prosthesis
- Contralateral testis biopsy: consider if atrophic testis, history cryptorchidism, or younger than 40yrs (36% risk ITGCN vs baseline 5-9% ITGCN in contralateral testis)
Testis-Sparing Surgery
- Indications: mass < 2-3cm, equivocal US findings, negative STM, solitary testis, and/or b/l tumors
- Obtain biopsies from normal adjacent tissue (look for ITGCN)
- Increased recurrence risk overall, especially local recurrence (11% vs 0%)
- If ITGCN present, offer XRT or orchiectomy due to higher risk of recurrence
- Risks: need for intraoperative orchiectomy, ipsilateral atrophy (3%), hypogonadism (7%)
Scrotal violation management
- Trans-scrotal biopsy and orchiectomy should not be performed
- Local recurrence risk: increases to 2.5% from 0%
- pT4 suspected: excise portion of scrotum en bloc during inguinal orchiectomy
- Scar excision after scrotal orchiectomy: 9% had residual disease
- Surveillance: perform groin exams, check inguinal/pelvic nodes on imaging
Management of Seminoma - 85% Stage I, 10% Stage II, 5% Stage III at presentation
Stage I
- Surveillance: 80-85% cured by orchiectomy (15-20% recurrence rate), most relapses occur within 2yrs (< 1% after 5yrs)
- Adjuvant treatment options: carboplatin x1 (recurrence 1-2%), XRT 20-30 Gy (recurrence 1-4%)
Stage II/III
- Lymphadenopathy < 3cm (Stage IIA): XRT 30 Gy (preferred), EP x4, BEP x3 (97% survival)
- Lymphadenopathy > 3cm (Stage IIB/C): EP x4, BEP x3, or XRT 36 Gy
Post-chemotherapy residual masses
- Residual mass > 3cm: residual disease in 30-50%, undergo FDG-PET/CT, if metabolically active consider biopsy/excision, template RPLND can be difficult due to post-chemo desmoplastic reaction
- Residual mass < 3cm: observation, unlikely to contain residual disease (0-4%)
- Necrosis in 90%, viable malignancy in 10%
- No benefit to XRT
- If viable tumor, give adjuvant chemo x2
Relapse
- No prior therapy: consider XRT (70-90% cure)
- Prior XRT: recommend chemotherapy (almost 100% cure)
- Prior carboplatin x1, give cisplatin based chemo
- Prior induction chemo, give second-line chemo (20-50% long-term survival), perform biopsy prior to chemotherapy (increased risk for teratoma)
- Late relapse is uncommon, consider cisplatin-based therapy
Management of NSGCT - 33% Stage I, 33% Stage II, 33% Stage III at presentation
Stage IA-IB
- Options: offer observation, BEP x1-2, or RPLND (both with almost 100% cure)
- Surveillance:20-30% relapse if IA, 40-50% relapse if IB
- Consider treating: if LVI and embryonal carcinoma present (> 45-90% primary), increased risk for relapse relapse (30-90% risk vs baseline < 20%)
- Timing:> 90% relapses occur within 2yrs, but 1-5% will occur 5+yrs later
- Somatic malignancy: if present in orchiectomy specimen, recommend RPLND (non-responsive to chemotherapy
Stage IS
- 37-100% eventually develop elevated STMs or RP metastases
- Management: induction chemotherapy, > 90% cancer-specific survival
Stage IIA
- Normal STMs: offer BEP x3, EP x4, or RPLND
- Teratoma present: recommend RPLND
- Elevated STMs or LN > 3cm, recommend chemotherapy
- Prior inguinal surgery: recommend chemo (risk of abnormal lymphatic drainage)
Stage IIB
- All patients: offer BEP x3 or EP x4
- RPLND alternative but most patients will need adjuvant chemo due to risk of relapse
Stage IIC + III
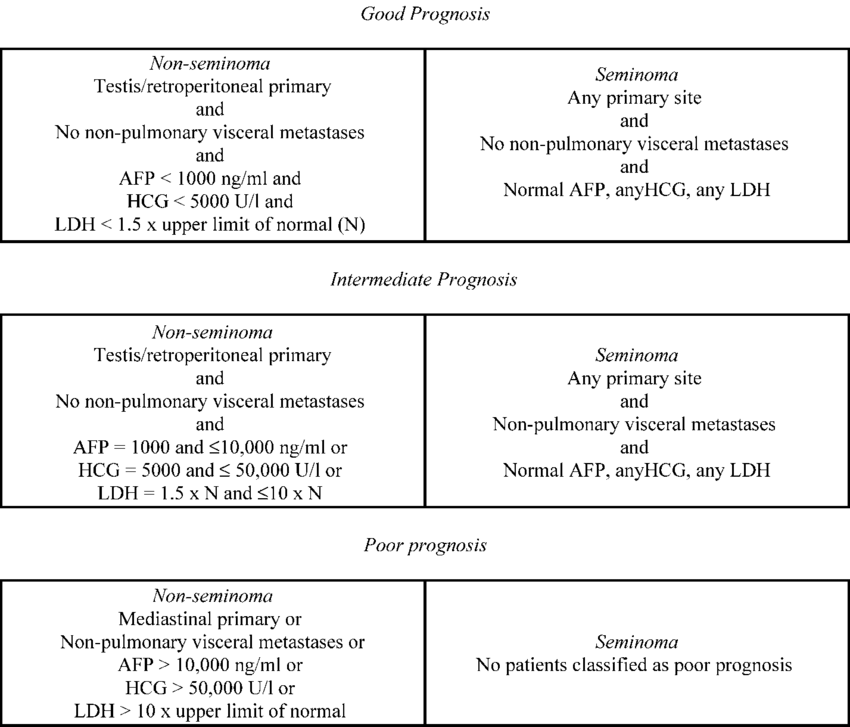
- All patients: induction chemotherapy, regimen based on IGCCCG classification
- Salvage chemo: give for post-chemo marker elevation or disease progression
- RPLND: only for post-chemo RP nodes > 1cm
- Teratoma may be present even if not seen in primary specimen
Post-chemotherapy residual masses
- Up to 40% contain residual teratoma
- Residual mass < 1cm: observe, unlikely to contain malignancy, only 9% recur (only 4% in RP)
- Residual mass > 1cm: full bilateral template RPLND
- Residual mass after salvage chemo: RPLND recommended no matter residual mass size, high risk for containing cancer (50%) or teratoma (40%)
Relapsed disease
- No prior chemotherapy: induction chemotherapy has 95% cure rate
- Prior chemotherapy: consider TIP, VIP, or high-dose chemo
- Prior chemotherapy with residual/new mass: recommend surgical resection
- Elevated STMs despite 1st/2nd line chemo: consider "desperation surgery" if potentially resectable single site, with 33-57% long-term survival
- Late relapse (> 2yrs): may be viable malignancy (54-88%), teratoma (12-28%), or malignant transformation (10-20%), usually managed with surgical resection (chemo-resistant)
Brain metastases
- Seen mainly with choriocarcinoma, mortality 4-10% due to hemorrhage
- Brain relapse worse prognosis than brain mets at initial diagnosis
- Management: BEP x4 then mass resection
Adjuvant therapies
Chemotherapy
- Primary options: EP x4 vs BEP x3 for good risk patients, BEP x4 for intermediate/poor risk patients, VIP x4 if contraindication to bleomycin (pulmonary concerns)
- Salvage options: VeIP, TIP, high-dose chemo + autologous SCT
- Cisplatin vs carboplatin: do not use carboplatin for good risk metastatic patients, can use carboplatin for Stage IA-IB seminoma
- General early toxicity: fatigue, myelosuppression, infection, neuropathy, hearing loss, decreased renal function, mortality (0-4%)
- General late toxicity: neuropathy (14-43%), Raynaud syndrome (20-45%), ototoxicity (20-40%), hypogonadism (20-25%), infertility, secondary malignancy, cardiac disease
- Post-bleomycin surgical management: prevent complications with aggressive monitoring, avoid over-hydration, use colloids, "judicious" crystalloids, keep FIO2 < ;0.25
| Chemo agent | Mechanism | Toxicity |
|---|---|---|
| Bleomycin | Binds/breaks DNA | Pneumonitis, pulmonary fibrosis |
| Etoposide | Binds DNA | Myelosuppression, mucositis, 0.8% leukemia risk |
| Cisplatin | Crosslinks DNA | Nephrotoxicity, neurotoxicity, hearing loss, cardiomyopathy |
RPLND
- Template limits: renal vessels superiorly, ipsilateral ureter crossing iliacs inferiorly, ipsilateral ureter laterally, aorta/common iliac inferior to IMA, and contralateral ureter (R side) vs vena cava (L side) above IMA
- Right modified template: remove right common iliac, paracaval, precaval, retrocaval, interaortocaval, preaortic, and retroaortic nodes
- Left modified template: remove left common iliac, paraaortic, preaortic, retroaortic nodes
- Bilateral template indications: Stage II NSGCT, suspicious intraoperative nodes, pcRPLND, somatic malignancy in primary
- Remove ipsilateral gonadal vessels and spermatic cord remnant
- Size cutoff of 1cm for primary RPLND has a high false negative rate, have increased suspicion for LN > 5-9mm in primary landing zone
- pcRPLND findings: 40% necrosis, 45% teratoma, 15% viable malignancy
- Use clinical judgement for unilateral vs bilateral RPLND for clinically negative nodes
- Use adjuvant chemotherapy if pN2-3 with viable tumor
- Ejaculatory dysfunction: common, caused by post-ganglionic sympathetic nerve and hypogastric plexus injury, nerve sparing preserves ejaculatory function 99% of the time
- Complications: anejaculation, chylous ascites (2%), renal vascular injury (3%), bowel obstruction (1-3%), mortality (0.3%, higher for pcRPLND)
Radiation (seminoma only)
- Success: prevents relapse in 96% Stage I seminoma
- Templates: abdominal retroperitoneum if seminoma IA/IB, add ipsilateral iliac nodes for IIA/IIB (can consider for IA/IB as well)
- Radiate inguinal scar or ipsilateral scrotum if scrotal violation or tumor spillage occurred during surgery
- Potential contraindications: prior XRT, inflammatory bowel disease, pelvic/ectopic kidney (very radiosensitive)
- XRT side effects: nausea/vomiting, ulcers (< 5%), diarrhea, oligospermia, secondary malignancy (18% at 25yrs)
Non-Germ Cell Tumors
Sex cord stromal tumors
- Make up 0.4-4% testis tumors, 90% benign, can only prove malignant if metastatic disease present
- Consider testis-sparing surgery if mass < 3cm
- Leydig cell tumor: majority (75-80%) stromal tumors, present with mass/pain, gynecomastia, impotence, decreased libido, infertility, check hormone labs (T, LH, FSH, estrogen), manage with orchiectomy, if 2+ malignant features then manage with RPLND (usually chemo/XRT resistant), 40% require T supplementation post-orchiectomy
- Sertoli cell tumor: may secrete estrogen/tesosterone, may present with gynecomastia, manage similar to Leydig cell tumors
- Granulosa cell tumor: rare, 7% prepubertal testis tumors (75% diagnosed within 1mo birth), usually cured with orchiectomy
- Gonadoblastoma: occur almost always in patients with dysgenic gonads and DSD, 40% risk for bilateral tumors, bilateral orchiectomy recommended
Other testis tumors
- Dermoid/epidermoid cyst: onion peel appearance without flow on ultrasound, unlike teratoma will not have ITGCN in surrounding tissue
- Rete testis adenocarcinoma: rare, presents with mass + hydrocele, 50% have metastatic disease, median survival 1yr, RPLND may be curative but chemo/XRT are not useful
- Adrenal rests: remnant adrenal tissue seen with CAH, usually presents with bilateral testis masses, steroid therapy causes regression/stabilization
- Lymphoma: testis primary seen in 1-2% lymphomas, more commonly a site of spread, usually presents age 50-60+, 35% have bilateral involvement, 25% have systemic symptoms, 10% have CNS involvement, manage with orchiectomy, require further chemotherapy, usually poor prognosis due to systemic disease
- ALL: testicle is site for relapse after chemotherapy, can diagnose with biopsy, orchiectomy not needed, manage with XRT 20Gy and include contralateral testis, poor prognosis due to associated systemic disease
Adnexal tumors
- Adenomatoid tumor: most common paratesticular tumor, 75% involve epididymis, managed with inguinal resection
- Cystadenoma: associated with VHL syndrome, multicystic lesions, may be bilateral
- Mesothelioma: painless mass with hydrocele, manage with radical inguinal orchiectomy and hemiscrotectomy, consider node dissection, poor survival for malignant disease (median < 2yrs)
- Sarcoma: explore any solid non-testicular scrotal mass via inguinal approach and perform biopsy, perform wide excision including orchiectomy, usually recur locally, consider XRT for liposarcoma, RPLND for non-liposarcoma, chemotherapy for RP metastasis
References
- AUA Core Curriculum
- Calaway, Adam C., et al. "Percentage of teratoma in orchiectomy and risk of retroperitoneal teratoma at the time of postchemotherapy retroperitoneal lymph node dissection in germ cell tumors." The Journal of Urology 206.6 (2021): 1430-1437.
- Stephenson, A. and T. Gilligan. "Neoplasms of the Testis." Campbell-Walsh Urology 12 (2020).
- Stephenson, Andrew, et al. "Diagnosis and treatment of early stage testicular cancer: AUA guideline." The Journal of urology 202.2 (2019): 272-281.
- Wieder JA: Pocket Guide to Urology. Sixth Edition. J.Wieder Medical: Oakland, CA, 2021.
- Wilkinson PM, Read G. International Germ Cell Consensus Classification: a prognostic factor-based staging system for metastatic germ cell cancers. International Germ Cell Cancer Collaborative Group. J Clin Oncol. 1997;15:594-603.