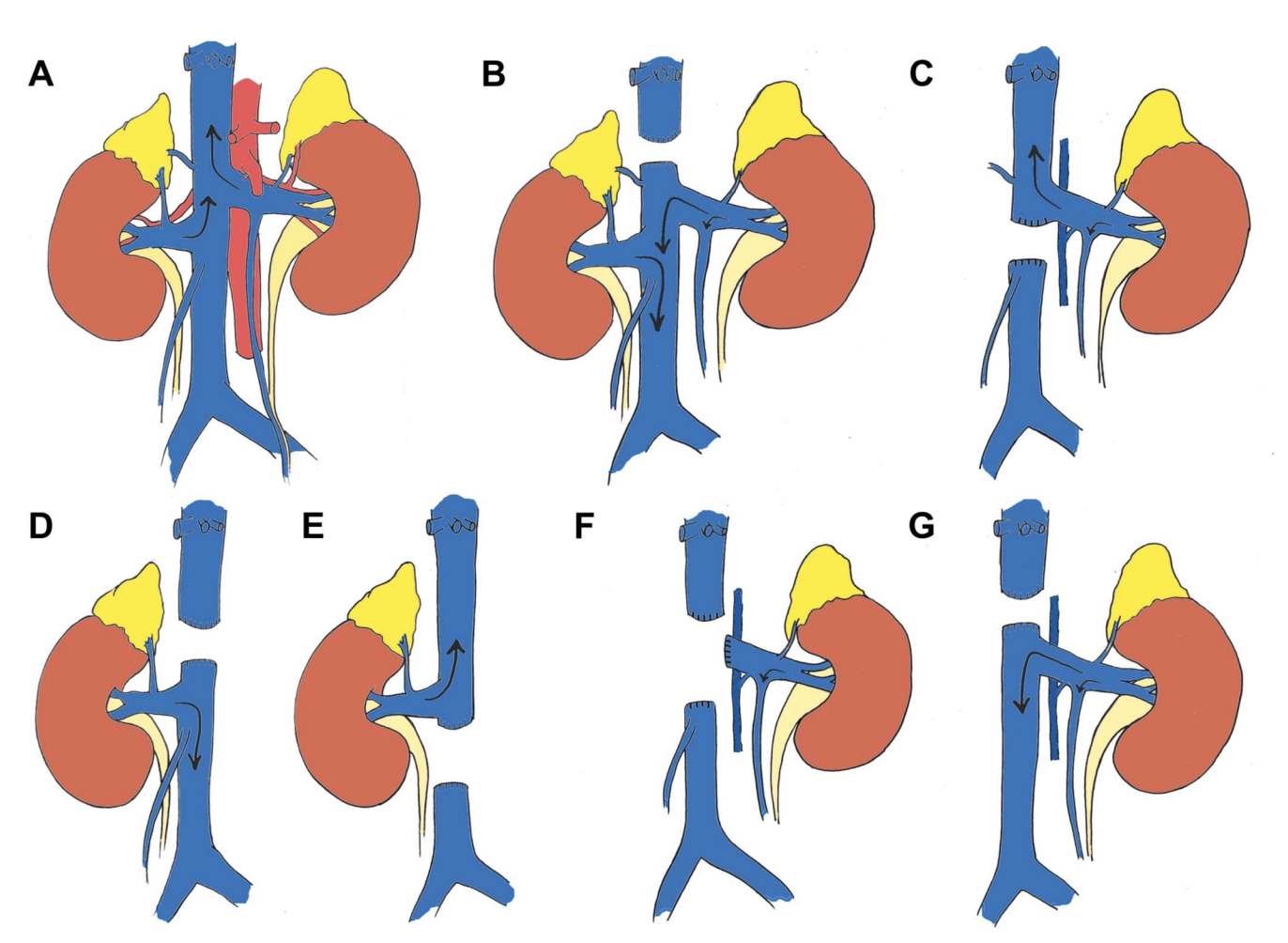
Kidney Cancer Treatment

TNM staging, from Campbell's
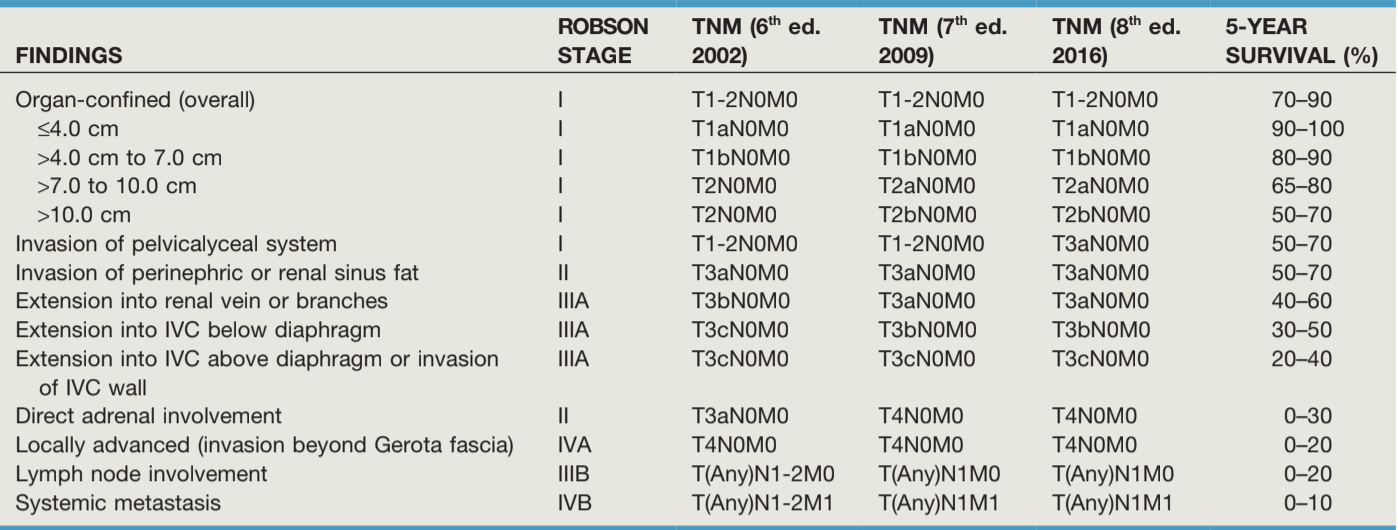
RCC Prognosis based on TNM staging, from Campbell's

Management recommendations, from AUA Guidelines
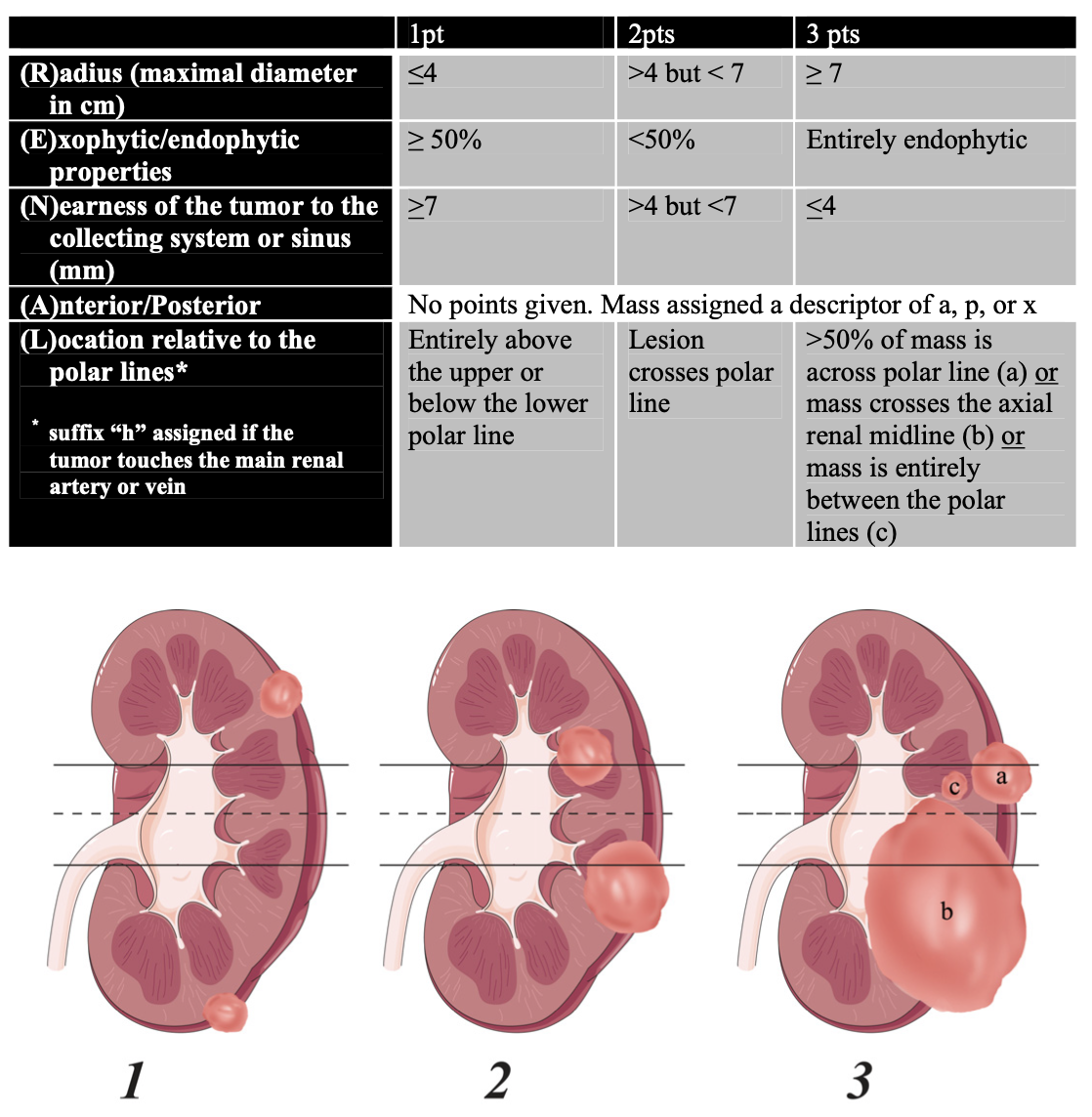
RENAL nephrometry score, from Kutikov 2009
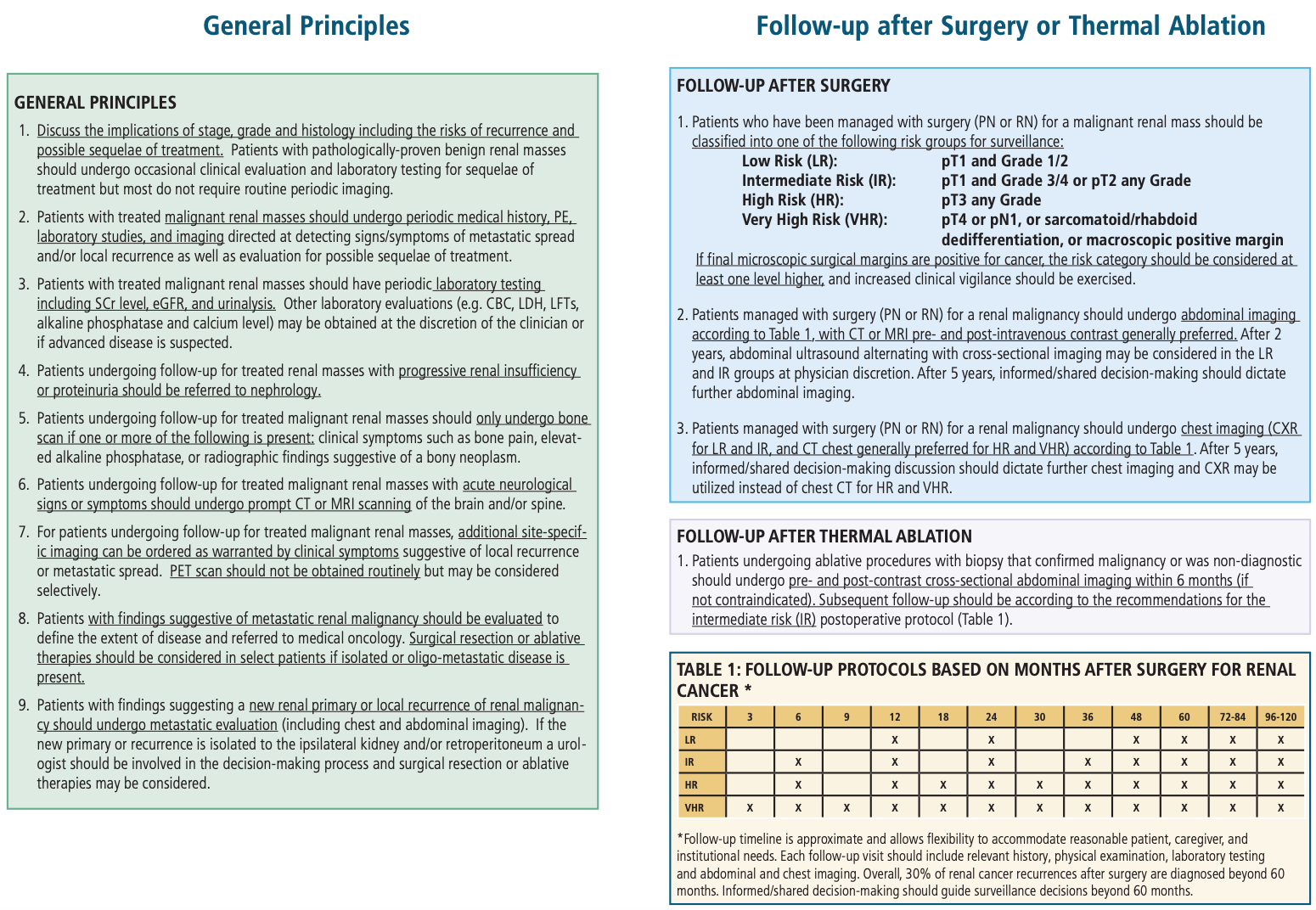
Post-treatment follow-up, from AUA Guidelines
Localized Kidney Cancer Treatment
Active Surveillance
- Indications: renal mass < 2cm (cT1a)
- Likelihood that mass is benign (nomogram): 35-45% if < 1cm, 20-25% if 1-2cm, 15-20% if 2-4cm, 10% if 4-6cm, 5% if > 6cm
- Risk of metastasis at 3yrs: 0% if < 2cm, < 2% if < 4cm
- Size matters: 20-30% < 4cm and 40% < 2cm are benign, most masses grow at 0.09-0.34cm/yr
- Good candidate: old/frail, life expectancy < 5yrs, high comorbidity or operative risk, poor functional status
- Bad candidates: for patients w/ > 2cm, higher stage, growth rate > 5mm/yr, changes in patient/tumor factors
- Biopsy: consider if findings would sway patient towards treatment
- Imaging: recommended q6-12 month intervals with CT, MR, or US, variation in size 3mm or less does not necessarily indicate interval growth, should also obtain chest imaging and labs annually
- Indications to treat: becomes > 2-3cm, interval growth > 0.5cm/yr, infiltrative appearance, change in clinical stage, becomes symptomatic, aggressive features on imaging
- Indolent lesions: subcentimeter masses with minimal growth over 5 years indicate very low risk for malignancy/metastasis, can discontinue surveillance after 5 years
Focal Ablation
- Criteria: cT1a < 3cm (100% success, vs 81% success if > 3cm)
- Anatomy selection: posterolateral tumor, > 0.5cm from UPJ or renal pelvis, > 1cm from bowel
- Overall survival at 5yr: 95% for < 3cm vs 79% for > 3cm, cancer specific survival 95-98%
- Biopsy: should be obtained at time of ablation
- Surveillance: assess with lack of contrast enhancement on post-treatment imaging, check at 3-6mo then annually for at least 5yrs
- Recurrence: 6% (vs 3% for PN or 1% for RN), can be treated with repeat ablation
- RadioFrequency (RFA): 460-500kHz leads to frictional heating, needs to reach 70 degrees for adequate kill (don't go above 105C can cause gas pockets), use multi-tine probe for improved delivery, more difficult to monitor effects in real time
- Cryoablation: temperatures below -20-40C leads to coagulation necrosis + edema during thaw, also causes thrombosis, form ice ball ~1cm outside renal mass to ensure adequate margin, perform double freeze/thaw cycle
- Temperature sink created by proximity to large vessels, can decrease efficacy of treatments
- Complications: overall 5-6%, hemorrhage 11-27%, transfusion 0-3%, obstruction, pneumo/hemothorax, renal failure, pancreatitis, lumbar radiculopathy
- Radiation: SBRT may be option for inoperable pT1a-b, with better local control than ablation above 4cm
Surgical Considerations
- AUA recommendation: masses > 2cm, surgical candidate, not ablation candidate
- Eventual need for dialysis: 1-12%
- Lymph node dissection: has not been shown to increase cancer-specific or overall survival
- GFR: tumor removal should have minimal effect on GFR (removed tissue is already abnormal/nonfunctional)
- Recurrence: 1-2%, cancer free survival 90+%
- Postop complication risk calculator
- Nephrometry score: helpful to predict tumor complexity, can use RENAL (see image), Padua
- Postop nomograms: UISS, MSK, SSIGN (clear cell only), ASSURE
- Timing for bilateral tumors: perform partial nephrectomy first (then contralateral radical nephrectomy), provides maximal renal parenchyma for recovery and decreased dialysis risk
- Adjuvant therapy: recommend MedOnc referral if concern for clinical metastasis or incompletely resected disease
Partial Nephrectomy
- Indications: solitary kidney (only 25-30% kidney required to avoid dialysis), bilateral tumors, familial RCC, CKD, proteinuria, risk for contralateral renal disease or tumors, younger age, multifocal tumors, comorbidities that may impact future renal function (HTN, DM, stones)
- Staging: best for cT1a, but can consider for cT1b-T2
- Enucleation: noninferior to obtaining margin, margin width nonrelevant, key factor is maintaining margin-negative disease
- Ischemia time: no clear cutoff but limit warm ischemia time as much as possible, some ischemia preferable to radical nephrectomy (infinite ischemia time)
- Positive margin: majority of patients will remain disease free, if radical nephrectomy is performed then residual disease found in < 16%
Radical Nephrectomy
- Indications: high tumor complexity, risky/challenging partial nephrectomy, no CKD/proteinuria, normal contralateral kidney
- Less complications than partial nephrectomy: bleeding (1% vs 3%), urine leak (0% vs 4%), reoperation (2% vs 4%)
- Adrenalectomy: recommended if concern for direct invasion or metastasis on either preoperative imaging or intraoperative findings, consider performing if the renal mass > 5cm or upper pole location, adrenal involved in < 10% RCC cases
Management of complicated localized RCC situations
Locally Invasive (T4)
- Prevalence on presentation: < 2%, may present with pain due to local invasion, although only 40% of suspected invasion are confirmed on pathology
- En bloc resection: recommended treatment, but ultimate prognosis is overall poor (10-20% survival at 12 months)
Clinical Node Disease (cN1)
- Prevalence: only 30-43% have pathology-confirmed nodal disease
- Likelihood based on pathologic size: 20% for 7mm, 29% for 10mm, 90% for 30mm
- > 40% nodal involvement if 2+ features: tumor > 10cm, cT3-4, tumor grade 3-4, sarcomatoid features, and histologic necrosis
- Lymphadenectomy: recommended if concerning lymphadenopathy, beneficial for disease staging but no proven survival benefit
- Templates: paraaortic and interaortocaval nodes on L, paracaval and interaortocaval nodes on R, remove from diaphragmatic crus down to common iliac artery
IVC Thrombus
- Prevalence: seen in 4-10% RCC
- Symptoms: lower extremity edema, right-sided varicocele, varicocele that does not collapse when supine, dilated abdominal veins, proteinuria, pulmonary embolus, right atrial mass, or nonfunctional kidney
- Staging: within renal vein (0), adjacent to renal vein ostium (I), extendeding below hepatic veins (II), intrahepatic below diaphragm (III), above diaphragm (IV)
- Bland thrombus: will not enhance on contrasted imaging, differentiating from tumor thrombus
- Anticoagulation: prophylactic dosing only, treatment dosing if bland thrombus present
- Preoperative renal artery embolization: not recommended, minimal proven benefit
- IVC repair options: suture cavoplasty (running prolene x2), patch cavoplasty (IVC narrowed > 30-50%, use autologous or bovine pericardium), IVC replacement (> 75% narrowed, PTFE ringed graft but prone to thromosis)
- IVC ligation/resection indications: extensive infrarenal bland thrombus (prevents massive VTE, usually has collateral circulation), IVC wall invasion (suspect if > 3.5cm IVC diameter or IVC wall signal loss on MRI, easier for R side as L kidney has collaterals)
- Success rates: 45-70% can be cured with nephrectomy + thrombectomy alone (no adjuvant therapy), but immediate postoperative mortality rates 5-10%, 1-2% risk of embolization to pulmonary arteries
- Palliative thrombectomy: may have some benefit even in poor surgical candidates due to treatment of bothersome symptoms
- Systemic therapy: can consider for large tumors to shrink enough to more safely perform surgery
Local Recurrence
- Prevalence: 2-4% of cases (1-10% for partial nephrectomy), more likely with locally advanced disease, node-positive disease, or adverse pathologic features
- Timing: 60-80% local recurrences occur simultaneously with metastatic disease, majority of other recurrences recur within 3yrs of initial resection
- Isolated recurrence: curable with local resection for 30-40%, but may be difficult due to lack of tissue planes
Metastatic RCC Management
Surgical management in setting of metastatic disease
- Timing of cytoreductive nephrectomy: controversial, consider up-front immunotherapy followed by nephrectomy if good response
- Oligometastatic disease: treat surgically whether synchronous or metachronous, brain/bone mets can be treated with XRT, better prognosis if initially M0 (metachronous) as opposed to M1 (synchronous mets), lung oligomets have better prognosis than other sites
- Nonoligometastatic cytoreductive nephrectomy indications: low/favorable risk candidates, especially if ECOG 0/1, no brain mets, and only mets are in the lung
- Palliative CRN indications: pain, hematuria, systemic symptoms, paraneoplastic symptoms
- Brain metastases: respond best to surgery or XRT
Current targeted therapy options
- anti-VEGF: sunitinib, sorafenib, pazopanib, axitinib, cabozantinib, lenvatinib, bevacizumab
- mTOR inhibitors: temsirolimus, everolimus
- anti-PD1: nivolumab, pembrolizumab
- anti-PDL1: avelumab
- anti-CTLA-4: ipilimumab
- TKI side effects: hand/foot syndrome, GI distress, elevated LFTs, hypothyroid, pancytopenia, stroke, MI, worse wound healing (stop prior to elective surgery)
- mTOR inhibitor side effects: rash, stomatitis, asthenia, GI distress, pancytopenia, elevated LFTs, hyperglycemia, hyperlipidemia, rare bowel perforation, rare interstitial pneumonitis
- mTOR origin: discovered in 1972 on Rapa Nui (Easter Island), Rapamycin (sirolimus) produced by Streptomyces hygroscopicus, originally created as antifungal then realized it had immunosuppresive and anti-neoplastic properties, mTOR = "mammalian/mechanistic target of rapamycin"
"Current" recommendations
- Treatment naive, good risk: targeted agent alone, or axitinib + pembrolizumab/avelumab
- Treatment naive, intermediate/poor risk: nivolumab + ipilimumab or axitinib + pembrolizumab/avelumab
- Failed sunitinib/sorafenib: everolimus
- Failed anti-VEGF therapy: everolimus + lenvatinib, nivolumab, cabozatinib
- Failed first-line therapy: axitinib
- non-ccRCC: refer for clinical trial, sunitinib, cabozatinib, everolimus +/- levnvatinib
MSK model for predicting outcomes with chemotherapy or cytokine therapy
- Predictors: Karnofsky score < 80, elevated LDH (> 1.5x normal), low Hgb, elevated calcium (> 10), lack of prior nephrectomy
- 0 risk factors = median overall survival 20mo
- 1-2 risk factors = median overall survival 10mo
- 3+ risk factors = median overall survival 4mo
IMDC model for predicting outcomes with VEGF-targeted therapy
- Predictors: Karnofsky score < 80, neutrophilia, low Hgb, elevated calcium (> 10), thrombocytolosis, < 1yr from diagnosis to starting VEGF therapy
- 0 risk factors = median overall survival 43mo
- 1-2 risk factors = median overall survival 23mo
- 3+ risk factors = median overall survival 8mo
Prior therapies (mainly historical interest)
- IFN-a: response rate 10-25%, durable response < 2%
- IL-2: response rate 15-20%, complete regression 7-9%
- IFN-a + IL-2: response rate 19%, no overall significant difference in survival
- Conventional chemotherapy: response rate 5-6%
- Hormone therapy: response rate 2%
References
- AUA Core Curriculum
- Campbell, C., B. Lane, and P. Pierorazio. "Malignant Renal Tumors." Campbell-Walsh Urology 12 (2020).
- Campbell, Steven C., et al. "Renal mass and localized renal cancer: evaluation, management, and follow-up: AUA guideline." The Journal of Urology (2021)
- Kutikov, Alexander, and Robert G. Uzzo. "The RENAL nephrometry score: a comprehensive standardized system for quantitating renal tumor size, location and depth." The Journal of urology 182.3 (2009): 844-853.
- Parker, W. and M. Gettman. "Benign Renal Tumors." Campbell-Walsh Urology 12 (2020).
- Tracy, C. and J. Cadeddu. "Nonsurgical Focal Therapy for Renal Tumors." Campbell-Walsh Urology 12 (2020).
- Wieder JA: Pocket Guide to Urology. Sixth Edition. J.Wieder Medical: Oakland, CA, 2021.
- Xie, Lillian, et al. "Outcomes in patients with renal cell carcinoma undergoing inferior vena cava ligation without reconstruction versus thrombectomy: a retrospective, case controlled study." The Journal of Urology 205.2 (2021): 383-391.